
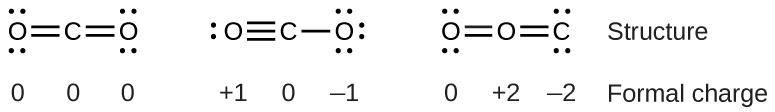
In chemistry, it is one of the most powerful oxidizing agents as it has a large standard reduction potential. The reason it is named “the ozone layer” is because of its high concentration of ozone, which absorbs the majority of the sun’s ultraviolet radiation. You have probably heard of the ozone layer in the earth’s stratosphere. Follow the octet rule and try to stay away from large formal charges.Įxample: How would you draw the resonance structures of Ozone (O 3)? What is Ozone?įirst, let’s take a look at ozone.Typically, you will be turning lone pairs into bonds and bonds into lone pairs.This means you will be pushing the electrons from the negative charge to the positive.
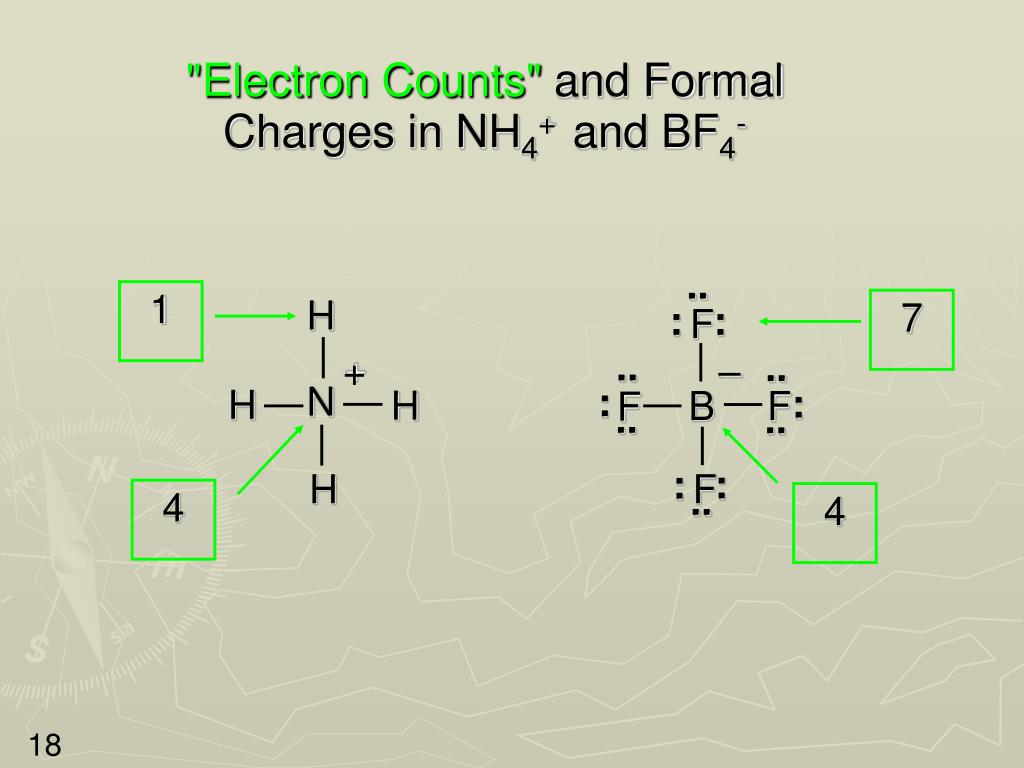
#Formal charge of carbon in h2co how to
You will also learn how to identify and compare the stability among these structures. In this tutorial on resonance structures, you will learn what resonance structures are and how to find all of the possible resonance structures a molecule has.


 0 kommentar(er)
0 kommentar(er)
